Leave Management for Biotechnology
Biotech leave management - ensure research continuity, maintain laboratory safety, and coordinate specialized expertise.
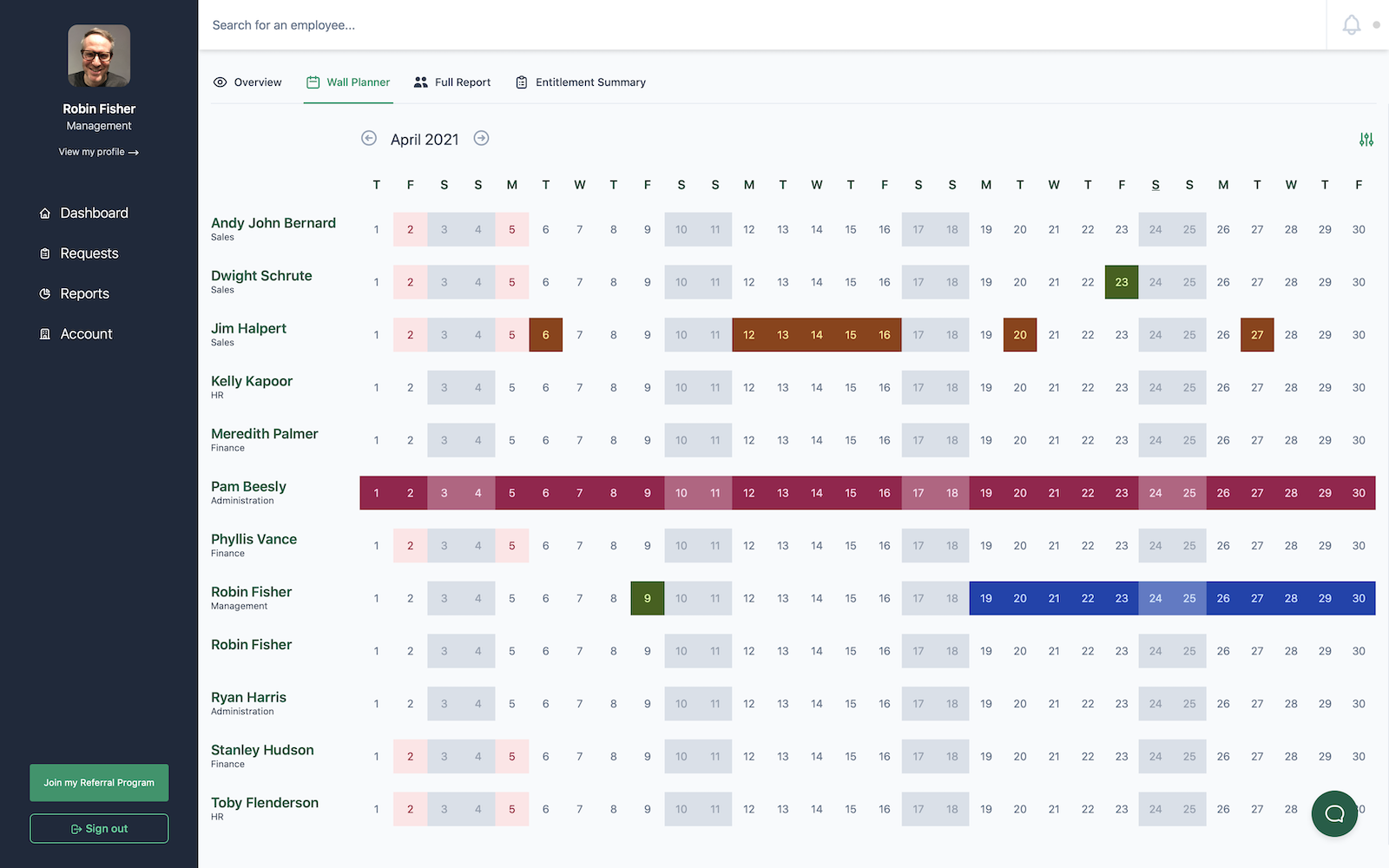
Biotechnology companies require specialized leave management that ensures research continuity and laboratory safety. Leavetrack helps biotech firms manage scientist and researcher absences while maintaining research progress and compliance standards.
Biotechnology Challenges
Biotechnology Industry Challenges
Biotech research requires specialized expertise and safety management
-
Research Project Continuity
- Biotechnology research involves complex experiments and studies that require continuous oversight and specialized expertise.
-
Laboratory Safety Compliance
- Biotech facilities must maintain strict safety protocols and specialized certifications for handling biological materials.
-
Regulatory Documentation
- Biotechnology companies must comply with FDA and regulatory requirements that require specific expertise and continuous oversight.
Solutions for Biotechnology
Research-Focused Biotech Solutions
Leavetrack optimizes biotechnology workforce management
-
Research Timeline Protection
- Project-based absence planning that considers research phases
-
Safety Certification Tracking
- Comprehensive monitoring of laboratory safety certifications
-
Specialized Expertise Coverage
- Intelligent coverage suggestions based on scientific expertise
Why Biotechnology Choose Leavetrack
Biotechnology companies using Leavetrack maintain research continuity and achieve 100% safety compliance. The platform's biotech-specific features ensure scientific progress while maintaining laboratory safety and regulatory standards.
- Maintained research continuity
- 100% safety compliance achieved
- Enhanced scientific collaboration
- Streamlined regulatory oversight
Ready to Transform Your Leave Management?
Start Your Free Trial Today
Join hundreds of biotechnology organizations already using Leavetrack to streamline their absence management.
